In nature, there is a magical element whose name is zinc. It is a silver-white and slightly bluish metal. It is the fourth largest consumer metal in the world, and one of the lifeblood of industry.

Interesting origin of the name zinc
The name zinc has an interesting origin, derived from the German word "zinke", which means "tooth", referring to the form zinc often took when smelted. It may in turn be derived from the Persian word "sing", meaning stone.
In the early 1500s, it was named zinc by Swiss physician Paracelsus, known and used for thousands of years.
What is special about zinc?
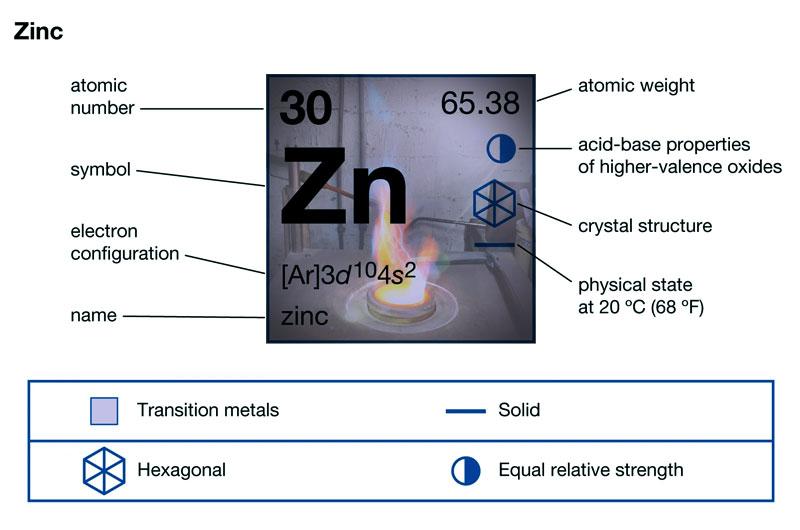
The element symbol for zinc is Zn, and what are the properties of zinc? It is a relatively soft metal with a Mohs hardness of 2.5 and has a metallic luster. Zinc is brittle, but its elasticity and ductility increase when heated to about 100 ° C.
Zinc metal has good wear resistance, castability, corrosion resistance, and good mechanical properties at room temperature. It is the third most widely used non-ferrous metal after aluminum and copper.
What ore is zinc extracted from?
Zinc is extracted from many different zinc ore minerals. Then what are the types of zinc ores?
There are various types of zinc ore in nature, but most of them exist as sulfides. The main zinc-bearing minerals are sphalerite, and a small number of oxidized minerals, such as smithsonite, hemimorphite, hydrozincite, etc.
Sphalerite
Sphalerite contains 67.1% zinc and usually contains iron, up to 30%. Those containing more than 10% iron are called marmatite. The difference in iron content directly affects the color of sphalerite. When iron increases, it changes from light to dark. Properties of sphalerite:

- Chemical composition: ZnS
- Hardness: 3.5 to 4
- Specific gravity: 3.9 to 4.1
- Crystal system: Equiaxed crystal system
- Luster: From diamond luster to semi-metallic luster
- Color: From yellow to light brown to dark brown, red, black, and colorless.
Sphalerite is the most widely distributed zinc mineral and is almost always associated with galena. In addition, it often contains rare elements such as manganese, cadmium, indium, and gallium. Therefore, sphalerite is not only the most important mineral raw material for extracting zinc, but also the raw material for extracting rare elements.
Smithsonite
Smithsonite is the most common zinc oxide mineral with high hardness and specific gravity. Similar to most carbonate minerals, it dissolves in hydrochloric acid and produces bubbles. Properties of smithsonite:

- Chemical composition: ZnCO3
- Hardness: 4 to 4.5
- Specific gravity: 4.3 to 4.45
- Crystal system: Triangular
- Luster: Vitreous or pearlescent
- Color: From yellow to light brown to dark brown, red, black, and colorless.
In addition to extracting zinc, it is translucent green or green-blue and can also be used as semi-precious jewelry. Smithsonite is also a traditional Chinese medicine, which has the effect of convergence and antisepsis.
Hemimorphite
Hemimorphite is located in the oxidation zone of lead-zinc sulfide deposits. It generally coexists with smithsonite, galena, limonite, etc. Properties of hemimorphite:

- Chemical composition: Zn4Si2O7(OH)2·H2O
- Hardness: 4.5 to 5
- Specific gravity: 3.4 to 3.5
- Crystal system: Orthorhombic
- Luster: Vitreous, sometimes pearly or diamond-like on the surface
- Color: Usually colorless or light blue, but also white, gray, light green, brown, etc.
Hydrozincite
Hydrozincite is a secondary mineral of sphalerite. Properties of hydrozincite:

- Chemical composition: Zn5(CO3)2(OH)6
- Hardness: 2 to 2.5
- Specific gravity: 3.5 to 4
- Crystal system: Monoclinic
- Luster: From silky to pearly or dull
- Color: From white to gray, light pink, light yellow, or brown.
Where is zinc found?
According to the zinc data of the United States Geological Survey (USGS): At present, the proven reserves of zinc resources in the world are about 1.9 billion tons, and the reserves of zinc ore are about 250 million tons.
In which countries is zinc found? The proportion of global zinc ore reserves is as follows:
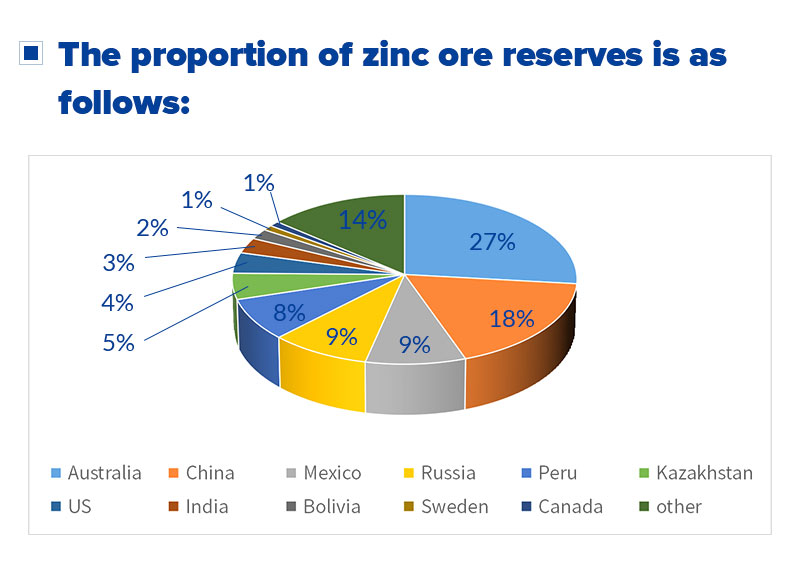
The country with the largest zinc reserves in the world is Australia, accounting for 27%, followed by China, Mexico, Russia, and so on. The ore reserves of these four countries account for about 63% of the world's zinc reserves.
What is zinc used for?
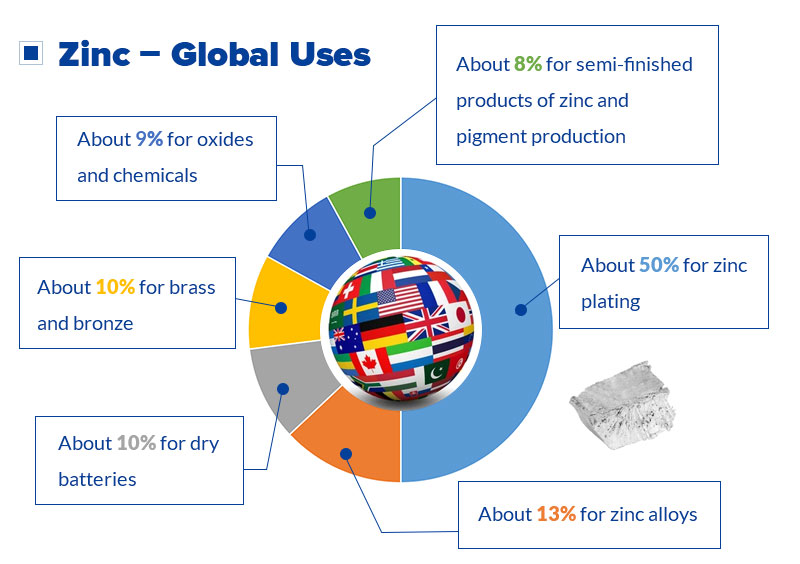
Zinc is a multi-functional metal in the world, called the lifeblood of industry. Various uses of zinc: In zinc products, about 50% for galvanizing; about 13% for zinc alloys; about 10% for brass and bronze; about 10% for dry batteries, and about 9% for oxides and chemicals. The rest is used for semi-finished products of zinc and pigment production.
1. Galvanizing

Because zinc tends to form a protective film on the surface at room temperature, exerting its corrosion resistance, the largest use of zinc is in the galvanizing industry.
It is mainly used for surface coating of steel (such as galvanized sheet), applied in automobile, construction, shipbuilding, light industry, and other industries.
2. Zinc alloy

The strength and hardness of zinc itself are not high. However, after zinc is added with alloying elements such as aluminum and copper, the strength and hardness are greatly improved. The most common alloy is brass, which is a mixture of 20%-45% zinc and copper.
Due to its superior superplastic properties, zinc alloys are widely used in the production of die-castings and parts in the automobile and machinery industries.
3. Battery

Zinc can be used to make batteries, such as zinc-manganese batteries and the latest zinc-air batteries.
Zinc-ion batteries are considered safer than lithium-ion batteries used in electric vehicles.
4. Zinc oxide

Zinc oxide is a white compound that is a commonly used chemical additive. It is widely used in rubber, paint, enamel, medicine, printing, fiber, and other industries.
Moreover, zinc oxide has natural anti-corrosion and anti-bacterial properties, which can protect the skin. It is also opaque to UV light and is an excellent sunscreen.
In addition to modern industry, zinc has an indelible position. For people, zinc is also one of the necessary trace elements for the human body, known as the spark plug of life or the flower of life. Because zinc can promote body development and tissue regeneration.
Conclusion
In a word, zinc is essential for industrial development and human development, so people cannot lack zinc. After learning zinc, do you want to get zinc? Zinc often coexists with lead, and you can read this blog to extract zinc from lead-zinc ore:
Ftmmachinery will provide you with zinc processing equipment and hold your economic lifeline!